Industria Farmacéutica y Biotecnología
Las empresas del sector farmacéutico y de biotecnología están entre las que son más fuertemente reguladas del mundo. La globalización, la creciente complejidad de las enfermedades, la introducción de nuevas tecnologías y las nuevas demandas tanto de pacientes, como de las agencias reguladoras, hacen que el ambiente reglamentar cambie constantemente.
Puede satisfacer todos los aspectos de las necesidades específicas de este sector, desde la gestión del ciclo de vida del producto hasta el seguimiento de los indicadores de rendimiento y la optimización de procesos, simplificando el cumplimiento y la gestión de la calidad.
soluciones para Pharma & Biotech
que reducen el coste de la conformidad normativa y auxilia a las empresas a potenciar el éxito, la productividad, a reducir los riesgos y a adherir a diversas normas.
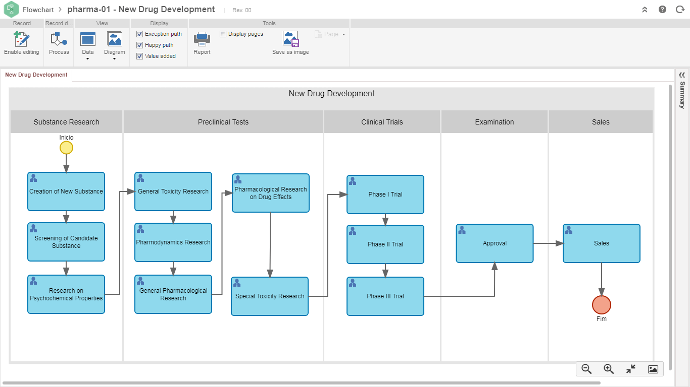
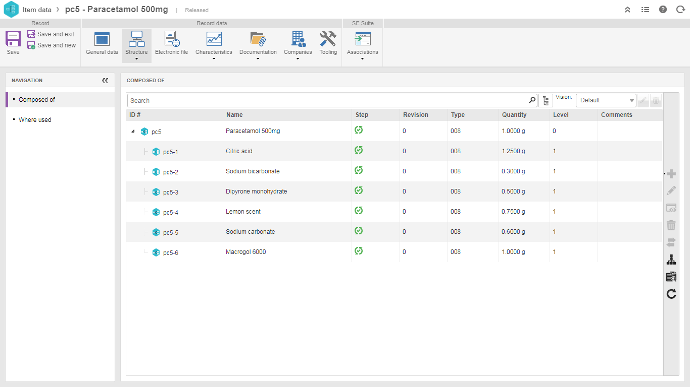
Permite a mapear, analizar y mejorar continuamente la eficiencia de las operaciones, integrando en una única plataforma diversas estructuras de gestión, incluyendo diseño de productos, asuntos regulatorios, eventos adversos, gestión de cambios, registro electrónico de lotes / Electronic Batch Record (EBR) y gestión de proveedores, entre otros
Ofrece a los equipos una visión de su rol en las actividades, desde el inicio hasta el fin. Ellos se vuelven más capacitados para reconocer las oportunidades, en vez de apenas resolver las situaciones críticas. El software ofrece una poderosa base de soporte para la colaboración, hasta fuera de los límites organizacionales, lo que les permite a los profesionales desempeñar un importante rol en la mejora de la forma en que la organización atiende a sus clientes.
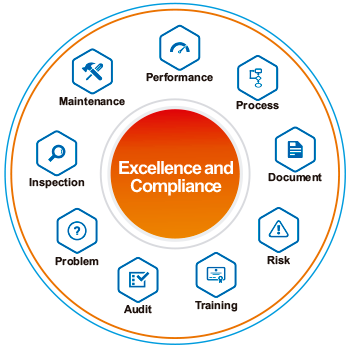
Simplifica y estandariza los procesos de gestión de excelencia y cumplimiento al proporcionar una plataforma en línea que funciona como un punto central de acceso para toda la documentación, KPI, políticas, plantillas, procedimientos y tareas actualizados.
- Administrar el desempeño corporativo desde el nivel estratégico al nivel operativo.
- Aumenta la conformidad regulatoria, a través de la elaboración y aprobación de documentos electrónicos, gestión de copias impresas y auditoría de sistema.
- Reducir los costes operativos.
- Mejorar la eficiencia operativa.
- Compartir de forma instantánea las informaciones de desempeño y calidad en toda la empresa.
- Estandarizar y simplificar los procesos farmacéuticos y biotecnológicos.
- Garantizar la comunicación corporativa a través de portales, alertas y notificaciones.
- Identificar, analizar, evaluar, monitorear y administrar los riesgos, automatizando los procesos de monitoreo de seguridad e identificación de niveles de alerta.
- Monitorear los informes de no conformidades en el proceso de gestión de la calidad para las investigaciones y acciones correctivas.
- Identifique, analice, evalúe, controle y gestione los riesgos mediante la automatización de los procesos de vigilancia de seguridad e identificación de señales.
- Realiza un seguimiento de los informes de no conformidades en el proceso de gestión de calidad para investigaciones y acciones correctivas.